Recently, Professor Zhang Qiang's team from the Department of Chemical Engineering at Tsinghua University published the research results on the bulk/surface interface structure design of lithium-rich manganese-based cathode materials for all-solid-state metal lithium batteries. They proposed an in-situ bulk/surface interface structure regulation strategy, constructed a fast and stable Li+/e−pathway, and promoted the practical application of lithium-rich manganese-based cathode materials in all-solid-state lithium batteries.
Batteries play a vital role in the modern energy field and have achieved great success in portable electronic devices, electric vehicles, and grid-scale energy storage applications. However, while improving the energy density of batteries, ensuring the safety of batteries is the key. With the rapid growth of the demand for improving the energy density of batteries, the traditional lithium-ion battery technology that relies on traditional cathode materials and organic electrolytes has encountered technical bottlenecks in long-term cycle stability, wide temperature range, and safety. Compared with traditional lithium-ion batteries, all-solid-state lithium batteries can break through the higher energy density limit. Due to its excellent energy density and safety characteristics, it has also become the most promising next-generation battery technology. Despite this, classic cathode materials cannot currently meet the high energy density and safety requirements of all-solid-state lithium batteries. Lithium-rich manganese-based cathode materials have become the most promising cathode materials for all-solid-state lithium batteries due to their discharge specific capacity≥250 mAh/g, energy density≥1000 Wh/kg, and low Co and Ni content.
However, due to the low electronic conductivity and obvious irreversible redox reaction, the interface structure is severely degraded, which makes the kinetic behavior of lithium-rich manganese-based cathode materials during charge and discharge impaired. Oxygen escape phenomenon exacerbates this interface failure behavior, leading to oxidative decomposition of the electrolyte, which in turn destroys the interface stability between lithium-rich manganese-based cathode materials and electrolytes.
Constructing and maintaining a stable Li+ and e−transport path for the battery in working state is the prerequisite for promoting the long cycle of all-solid-state batteries under practical conditions. The research team can construct a stable and fast Li+/e−pathway in situ at the cathode material/solid electrolyte interface by adjusting the bulk/surface interface structure and innovative design, promote the redox reaction activity of anionic oxygen, and enhance the reversibility of the redox reaction of anionic oxygen on the surface of the cathode material of all-solid-state lithium battery at room temperature, thereby stabilizing the high voltage solid-solid interface.
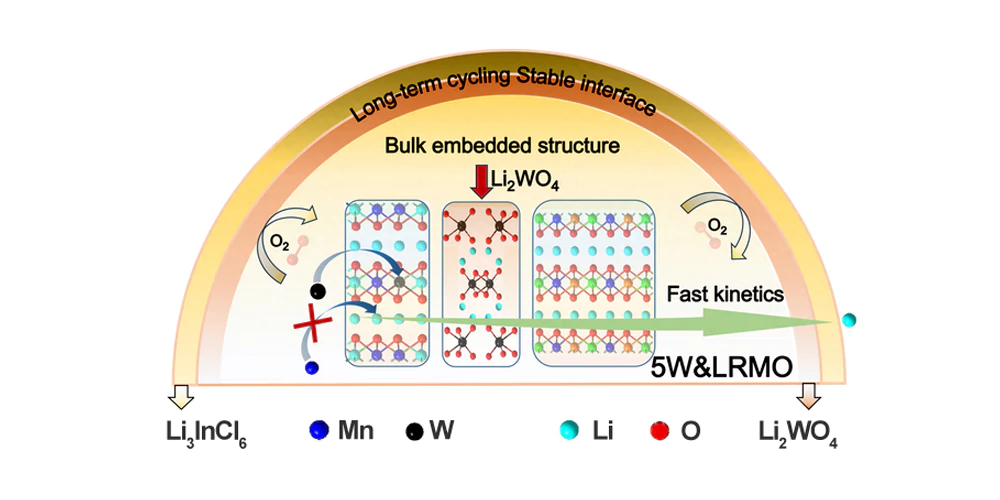
Figure 1. Schematic diagram of the modification of bulk/surface interface structure design strategy of lithium-rich manganese-based cathode materials
This study proposed a one-step synthesis strategy to optimize the bulk/surface interface structure of lithium-rich manganese-based cathode materials, and created a lithium-rich manganese-based cathode material (5W&LRMO) with a bulk embedded structure, W doping and Li2WO4 surface coating. This structure enhances the bulk structural stability of lithium-rich manganese-based cathode materials, improves the transfer kinetics of Li+/e−, and significantly enhances the redox activity of transition metal cations and anionic oxygen. Charge compensation of anionic oxygen redox reactions during the charge and discharge process is achieved, thereby promoting the reversibility of oxygen ion redox reactions on the surface of lithium-rich manganese-based cathode materials and stabilizing the high-voltage solid-solid interface. The optimized interface ensures charge and discharge stability in the high voltage range and maintains efficient Li+/e−transfer kinetics over a long cycle period, thereby improving the utilization rate of active substances in the composite cathode material.
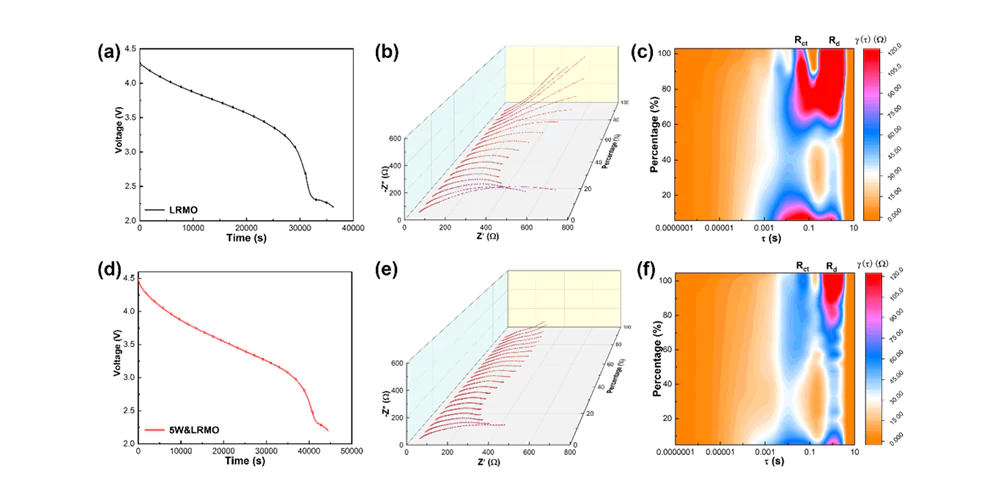
Figure 2. Evolution of interfacial Li+ transport kinetics of lithium-rich manganese-based cathode materials during the first charge and discharge process
This study revealed the impedance evolution process of the interface between the lithium-rich manganese-based cathode and the electrolyte by in-situ impedance spectroscopy (EIS) testing combined with relaxation time analysis (DRT). The proposed method enables visualization of the interface evolution process during the first charge and discharge and long cycle process. The study deeply understands the interface structure evolution between the lithium-rich manganese-based cathode material and the electrolyte before and after modification. It is found that the lithium-rich manganese-based cathode material before modification exhibits irreversible anion oxygen redox reaction at high voltage, further oxidizing the cathode and electrolyte interface, resulting in a significant increase in impedance and hindering the interfacial Li+ transmission. In contrast, the modified lithium-rich manganese-based cathode material exhibits stable/fast Li+ diffusion kinetics, especially at a high voltage of 4.6 V, minimizing the change in interfacial impedance value. Therefore, faster and more stable interfacial Li+ transmission is promoted by improving the reversibility of anion oxygen redox reaction. It is easier for composite cathode materials to achieve industrial-grade applications with a surface capacity of ~3 mAh/cm2 or even higher. At 25°C, the surface capacity of the high-area-load 5W&LRMO cathode material at 0.2 C rate is about 2.5 mAh/cm2, and the capacity retention rate is 88.1% after 100 cycles; at a high rate of 1 C, it shows ultra-long cycle stability, with a capacity retention rate of 84.1% after 1200 cycles. The research provides a new way to design the bulk/surface interface structure of lithium-rich manganese-based cathode materials and an effective way to improve the energy density of all-solid-state lithium batteries.
On October 1, the relevant research results were published in the Journal of the American Chemical Society under the title“Bulk/Interfacial Structure Design of Li-Rich Mn-Based Cathodes for All-Solid-State Lithium Batteries”.
TOB NEW ENERGY provides a full set of solid-state battery solutions, including solid-state battery materials, solid-state battery equipment, and solid-state battery production line solutions.