WANG Kunpeng ,1, LIU Zhaolin2, LIN Cunsheng2, WANG Zhiyu ,1,2
1. State Key Lab of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, China
2. Branch of New Material Development, Valiant Co., Ltd., Yantai 265503, China
Abstract
In comparison to Li-ion batteries, Na-ion batteries offer the benefits of low cost, good low-temperature performance, and safety, attracting great attention in the cost- and reliability-sensitive applications. With high capacity and low cost, Prussian blue-like materials (PBAs) stand as promising cathode materials for Na-ion batteries. However, the presence of crystalline water within their structure induces fast performance decay of the battery, serving as a critical bottleneck limiting their application. This work reports a facile thermal treatment strategy to effectively remove crystalline water from PBAs cathode materials, improving capacity retention from 73% to 88% after 340 cycles. The in-situ analysis uncovers that the initial loss of Coulombic efficiency of PBAs cathode is a result of its irreversible transformation from a trigonal form to cubic phase during the charging and discharging process. This issue can be addressed by introducing of Na2C2O4 to compensate the irreversible Na loss in the cathode. On this basis, a high-performance quasi-solid-state Na-ion battery is built by pairing a low-water-content PBAs cathode with Na2C2O4 additive and a hard carbon (HC) anode within a poly(ethylene glycol) diacrylate (PEGDA)-based quasi-solid-state electrolyte with high ionic conductivity and electrochemical stability. This battery exhibits the specific capacities ranging from 58 to 105 mAh·g-1 at current densities from 20 to 500 mA·g-1, capable of sustaining stable cycling for over 200 cycles. This study underscores the significant improvement in stability and capacity of PBAs cathode materials by the efficient removal of crystalline water in them.
Keywords: Na-ion battery; quasi-solid-state battery; Prussian blue cathode; in-situ analysis
The development of high-performance battery technology is a major strategic need for China to transform and upgrade its energy structure, promote a low-carbon clean economy, and achieve the goal of "carbon neutrality and carbon peak". Lithium-ion batteries are one of the most widely used high-efficiency battery systems. However, the abundance of lithium in the earth's crust is only 0.0065%, China's lithium resource reserves account for only 7% of the world's total, and nearly 70% of lithium carbonate is imported. It will be difficult to meet the huge demand in the field of energy storage and power batteries in the future. The abundance of sodium in the earth's crust is more than 400 times higher than that of lithium. China's sodium reserves account for about 22% of the world's total reserves. From the perspective of raw material costs, the cost of sodium-ion batteries can be reduced by 30% to 40% compared to lithium-ion batteries. In addition, compared with lithium-ion batteries, sodium-ion batteries have better low-temperature performance, a wider operating temperature range, and higher safety. These unique advantages have made them a focus of attention in cost-sensitive and safety-critical energy storage applications [1].
Driven by the "dual carbon" goal, China's demand for energy storage and power batteries reached 158.5 GWh in 2020, and the world's demand for batteries is expected to enter the TWh era in 2025. With the advancement of battery technology, the energy density of batteries has increased rapidly, and the requirements for battery safety have become increasingly prominent. Traditional lithium/sodium ion batteries use liquid organic electrolytes that are prone to leakage, which reduces the reliability of the battery [2-3]. The use of solid-state batteries with high thermoelectric stability, high mechanical strength, and no leakage risk is a feasible direction to solve the reliability problem [4-5], but it has problems such as high density of solid electrolytes, low ion conductivity, and poor contact with the "solid-solid" interface of electrodes [6]. Quasi-solid electrolytes between liquid and solid have better stability and safety than liquid electrolytes, and are superior to solid electrolytes in terms of ion conductivity, flexibility and interface compatibility [7⇓-9]. These advantages make quasi-solid-state batteries based on them one of the more feasible focus directions in the field of advanced battery technology.
Prussian blue compounds (PBAs) are currently the most popular cathode materials for sodium-ion batteries. Their open skeleton structure and abundant sodium storage sites give them a high theoretical specific capacity (170 mAh g-1) and good ion transport performance [10-11]. In solid-state batteries, PBAs can be used not only as cathode materials but also as solid electrolytes [12-13]. However, PBAs are generally prepared by solution precipitation, which will form Fe(CN)64- vacancy defects and a large amount of crystal water in their structure, hindering the embedding of Na+ into the PBAs lattice and limiting their sodium storage capacity. In addition, the crystal water in PBAs will gradually release into the electrolyte during the battery reaction, leading to rapid battery performance decay, side reactions, flatulence and other problems [11,14]. These problems limit the application of PBAs in solid-state batteries and make it difficult to match them with most water-sensitive inorganic solid electrolytes. The formation of vacancy defects and crystalline water in PBAs can be effectively inhibited by strategies such as hydrothermal treatment[15], slow coprecipitation[16], inhibition of Fe2+ oxidation[17], chemical etching[18], and element doping[19-20]. However, the relevant technical processes are complex and difficult to precisely control, and the performance of the obtained PBAs cathodes also needs to be improved. In view of the above problems, this study proposes a simple and efficient heat treatment method to reduce the content of crystalline water in PBAs and improve their sodium storage stability. Through in-situ polymerization technology, a polyethylene glycol diacrylate (PEGDA) benchmark solid electrolyte with high ionic conductivity and high electrochemical stability was developed. On this basis, the low-water content PBAs cathode and the hard carbon (HC) anode were matched in the PEGDA benchmark solid electrolyte, and Na2C2O4 was added to the cathode as a self-sacrificial sodium compensator to construct a high-performance quasi-solid-state sodium ion battery. The dynamic sodium storage mechanism of the PBAs cathode and HC anode was revealed by in-situ analysis technology.
1 Experimental method
1.1 Preparation of low water content PBAs cathode
116 mmol sodium citrate and 24 mmol FeSO4·7H2O were dissolved in 400 mL deoxygenated deionized water. 116 mmol sodium citrate and 26 mmol Na4Fe(CN)6 were dissolved in 400 mL deoxygenated deionized water. The solution containing FeSO4 was slowly added to the solution containing Na4Fe(CN)6, and the reaction was stirred at constant temperature for 6 h. The product was washed three times by centrifugation with ethanol and deoxygenated deionized water, and dried in a vacuum at 120 ℃ for 24 h to obtain PBAs with high crystalline water content (Hw-PBAs). It was placed in an argon-protected tube furnace and calcined at 270 ℃ for 2 h to obtain low-water content PBAs (Lw-PBAs), with a heating rate of 0.5 ℃·min-1.
1.2 Sample characterization
The sample morphology and structure were analyzed using a field emission scanning electron microscope. The sample chemical composition was analyzed using an X-ray photoelectron spectrometer and an inductively coupled plasma emission spectrometer. The battery was analyzed in situ using a powder X-ray diffractometer and laser Raman spectroscopy. The sample crystal water content was analyzed using a thermogravimetric analyzer in an argon atmosphere at a heating rate of 10 ℃·min-1.
1.3 Battery assembly and electrochemical performance testing
1.3.1 Liquid sodium ion half-battery assembly
CR2016 button cells were assembled for testing. Prussian blue cathode material (Hw-PBAs or Lw-PBAs), Ketjen black (KB) and polyvinylidene fluoride (PVDF) binder were uniformly mixed in a mass ratio of 8:1:1, N-methylpyrrolidone (NMP) was added as a solvent and dispersant, and the resulting slurry was uniformly coated on carbon-coated aluminum foil as the cathode, with an active material loading of 3~4 mg·cm-2. Metal sodium sheets were used as counter electrodes and reference electrodes. The electrolyte was a DMC/EC solution (DMC: dimethyl carbonate, EC: ethylene carbonate, volume ratio 1:1) of 1.0 mol·L-1 NaClO4 and 5.0% fluoroethylene carbonate (FEC). The battery was assembled in an argon-filled glove box (water content <10-7, oxygen content <10-7).
1.3.2 Liquid sodium ion full battery assembly
The positive electrode was prepared using the above method, HC was used as the negative electrode, and the N/P ratio of the positive and negative electrodes was controlled at 1.1~1.2. The battery was assembled in a glove box filled with argon (water content <10-7, oxygen content <10-7) using the above electrolyte.
1.3.3 Preparation of quasi-solid electrolyte
PEGDA was mixed with the above liquid electrolyte at a mass ratio of 7:93. 5.0% azobisisobutyronitrile (AIBN) was added as a polymerization initiator to form a precursor solution of quasi-solid electrolyte. This solution was heated at 60 °C for 10 h to form a quasi-solid electrolyte.
1.3.4 Assembly of quasi-solid-state sodium-ion full battery
The positive electrode material, Na2C2O4 sodium supplement, KB conductive agent and PVDF binder were uniformly mixed in a mass ratio of 6.4: 1.6: 1.0: 1.0, NMP was added as a solvent and dispersant, and the obtained slurry was uniformly coated on a carbon-coated aluminum foil as a positive electrode, with an active material loading of 3~4 mg·cm-2. HC was used as the negative electrode, and the positive and negative electrode N/P ratio was controlled at 1.1~1.2. The precursor solution of the quasi-solid electrolyte was added to the battery, and after the battery was encapsulated, it was heated at 60 ℃ for 10 h to obtain a quasi-solid-state battery. The battery was assembled in a glove box filled with argon (water content <10-7, oxygen content <10-7).
1.3.5 Battery performance test
The ionic conductivity of the quasi-solid electrolyte was tested by electrochemical impedance spectroscopy (EIS) using an electrochemical workstation. The test frequency range was 1 Hz~1000 kHz and the perturbation voltage amplitude was 5.0 mV. The electrochemical stability window of the quasi-solid electrolyte was tested by linear sweep voltammetry (LSV) with a sweep rate of 5 mV·s-1. The material and battery performance were studied by constant current charge and discharge method using a Land CT2001A battery tester. The half-cell voltage window was 2.0~3.8 V (vs. Na/Na+), the full-cell voltage window was 1.5~ 3.8 V, and the current density was 10~500 mA·g-1. When testing the cycle stability, the battery was first cycled 5 times at a current density of 50 mA·g-1, and then the cycle stability test was performed at different current densities.
2 Results and Discussion
2.1 Morphology and composition analysis
The TGA curve of Hw-PBAs in Figure 1(a) shows two regions of rapid weight loss: 1) room temperature to 270 °C, 2) 440 to 580 °C. In the former region, the weight loss from room temperature to 120 °C (mass fraction 3.1%) is caused by the removal of adsorbed water; the weight loss from 120 to 200 °C (mass fraction 6.10%) is caused by the removal of interstitial water in the PBAs framework structure; the weight loss from 200 to 270 °C (mass fraction 6.89%) corresponds to the removal of crystal water in PBAs. Therefore, 270 °C heat treatment was selected to remove water from Hw-PBAs. After heat treatment at this temperature, the obtained Lw-PBAs only lost about 1.18% of their weight at room temperature ~270 ℃, which was 92.67% lower than that of Hw-PBAs; and lost about 0.74% of their weight at 200~270 ℃, which was 89.26% lower than that of Hw-PBAs. The above results show that heat treatment can effectively remove different types of water in PBAs, and the obtained low-water content PBAs have good thermal stability.
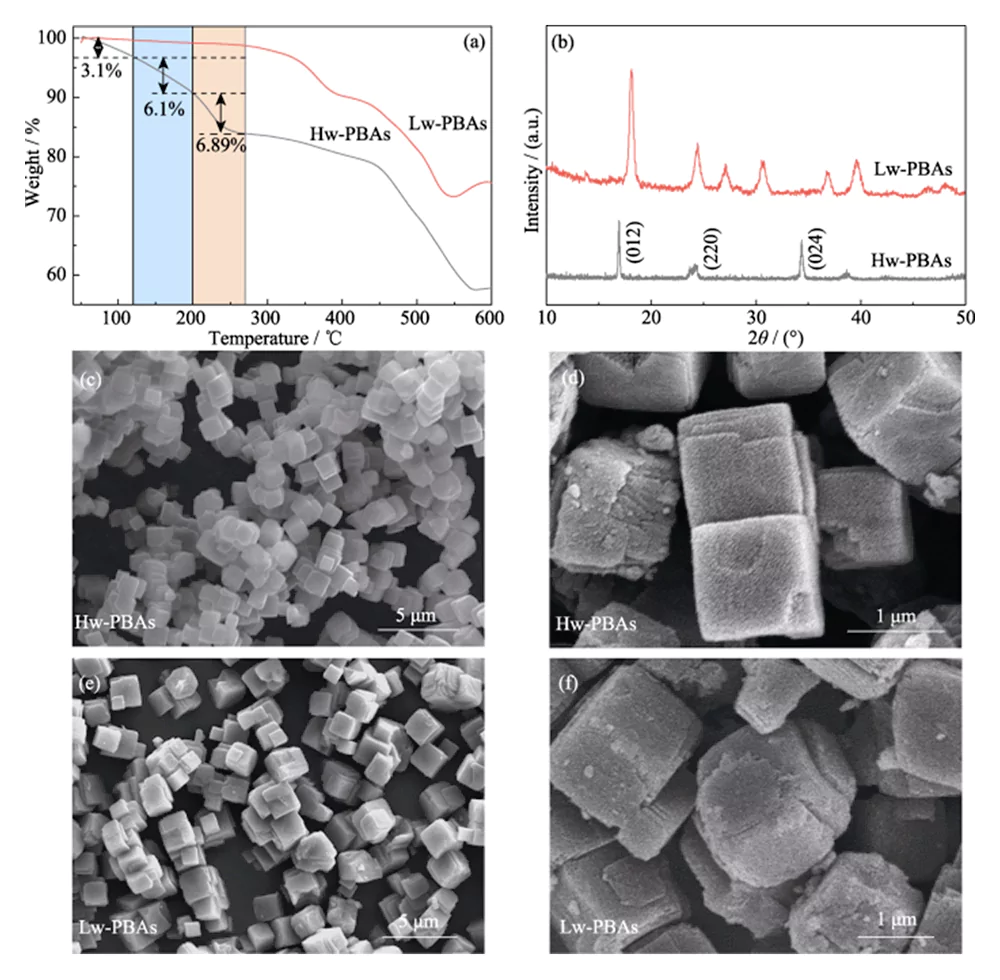
Fig. 1 TGA, morphology and structure analyses of PBAs cathode
(a) TGA curves and (b) XRD patterns of Hw-PBAs and Lw-PBAs; (c-f) SEM images of (c, d) Hw-PBAs and (e, f) Lw-PBAs
Figure 1(b) shows the XRD spectra of Hw-PBAs and Lw-PBAs. The diffraction peaks of Hw-PBAs at 2θ=17.0°, 24.0°, and 34.4° correspond to the (012), (220), and (024) crystal planes, respectively. After heat treatment, the diffraction peak corresponding to the (024) crystal plane disappears, indicating that the crystal water is successfully removed, and the diffraction peak positions corresponding to the (012) and (220) crystal planes move to higher angles, indicating that the unit cell volume decreases after the crystal water is removed. In addition, new diffraction peaks appear at 2θ=27.1°, 30.7°, and 36.9°, indicating that a trigonal crystal structure is formed after heat treatment. SEM analysis (Figure 1(c~e)) shows that Hw-PBAs and Lw-PBAs have similar cubic morphologies with an average size of 2~3 µm. The surface of the Lw-PBAs particles obtained after heat treatment is slightly rough (Figure 1(f)), but due to the low heat treatment temperature, no obvious melting and agglomeration occurred. The composition of Lw-PBAs was estimated to be Na1.91Fe- [Fe(CN)6]·3.2H2O by analyzing the metal element content by ICP-OES and measuring the water content by TGA.
To further explore the chemical composition and structure of Hw-PBAs and Lw-PBAs, XPS analysis was performed. In the high-resolution Fe2p XPS spectrum of Hw-PBAs, the two characteristic peaks at binding energies of 708.6 and 721.4 eV correspond to Fe(II) and Fe(III), respectively (Figure 2(a)). Fe(II) and Fe(III) also exist in Lw-PBAs, but the proportion of Fe(III) increases significantly (Figure 2(b)). This is because [NaH2O]+ is removed from the PBAs structure during the heat treatment process, and Fe(II) in Lw-PBAs is partially oxidized to maintain valence equilibrium. In the high-resolution O1s XPS spectrum of Hw-PBAs, the characteristic peaks at binding energies of 536.0, 533.7, 531.9 and 529.7 eV correspond to interstitial water, coordinated water, surface hydroxyl groups and oxygen species in the PBAs lattice, respectively (Figure 2(c)). After heat treatment, the characteristic peak corresponding to coordinated water disappears, indicating that this process can effectively remove coordinated water from Lw-PBAs (Figure 2(d)). During this process, Fe on the surface of PBAs reacts with hydroxyl groups to form iron oxides, causing the Fe-O characteristic peak at the binding energy of 530.0 eV to be greatly enhanced.
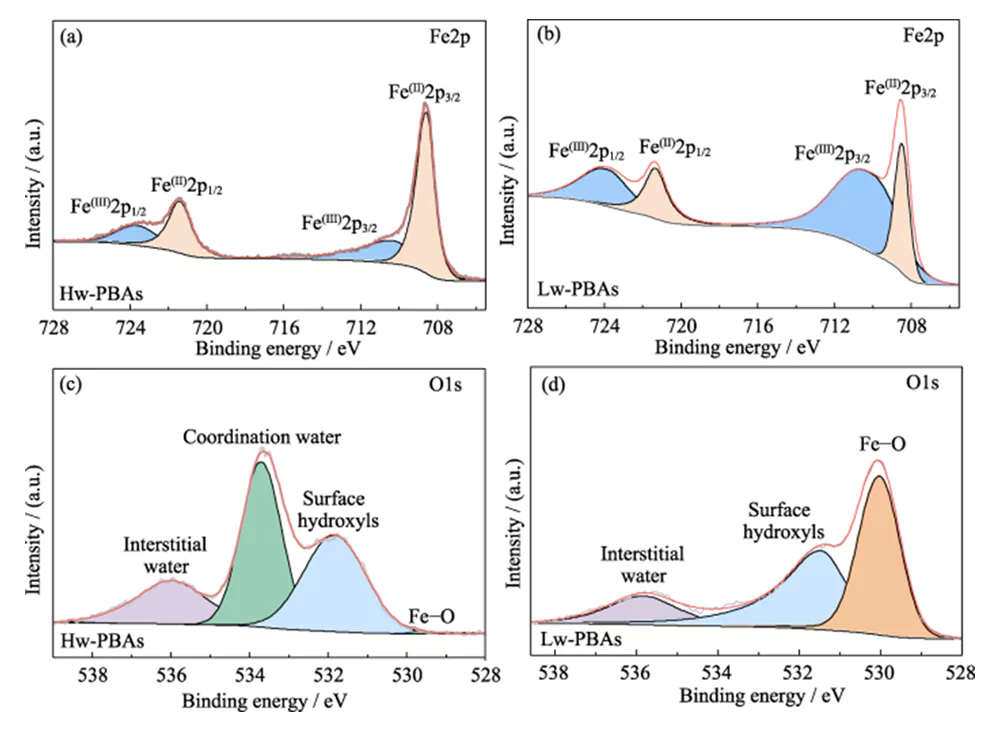
Fig. 2 Chemical composition of PBAs cathode
(a, b) Fe2p XPS spectra of (a) Hw-PBAs and (b) Lw-PBAs; (c, d) O1s XPS spectra of (c) Hw-PBAs and (d) Lw-PBAs
2.2 Electrochemical performance
Figure 3(a) shows the constant current charge-discharge cycle curves of sodium ion half-cells with Hw-PBAs and Lw-PBAs as positive electrodes at a current density of 100 mA·g-1, with a voltage window of 2.0~3.8 V (vs. Na/Na+). After 340 charge-discharge cycles, the Lw-PBAs positive electrode can still maintain a high specific capacity of 91 mAh·g-1, with a capacity retention rate of 88%, and an average single charge-discharge capacity loss rate of only 0.035%, showing excellent cycle stability. Under the same charge-discharge conditions, the capacity retention rate of the Hw-PBAs positive electrode without the removal of crystal water is only 73%, showing the important role of removing crystal water in improving the cycle stability of the PBAs positive electrode. Figure 3(b) shows the constant current charge-discharge curve of the Lw-PBAs cathode at a current density of 100 mA·g-1, showing a typical dual voltage platform feature: (1) The voltage platform of about 3.2 V corresponds to the redox process of low-spin Fe2+/Fe3+ (coordinated with C); (2) The voltage platform of about 2.9 V corresponds to the redox process of high-spin Fe2+/Fe3+ (coordinated with N). The appearance of a voltage platform at about 3.2 V indicates that the removal of crystal water is beneficial to strengthening the redox reaction of low-spin Fe2+/Fe3+ in PBAs, which helps to improve its sodium storage capacity. In the subsequent cycle process, the charge-discharge curve of the Lw-PBAs cathode remained basically consistent, showing good structural stability. At current densities of 10, 50, 100, 200, and 500 mA·g-1, the Lw-PBAs cathode can maintain high reversible specific capacities of 126, 112, 110, 108, and 107 mAh·g-1 (Figure 3(c)). In particular, at a high current density of 500 mA·g-1, the Lw-PBAs cathode has excellent capacity retention, and its specific capacity is about 13.4% higher than that of Hw-PBAs. When the current density drops back to 10 mA·g-1, the specific capacity of the Lw-PBAs cathode can be restored to 125 mAh·g-1, which is close to the initial specific capacity, indicating that it can maintain excellent structural stability during rapid sodium storage.
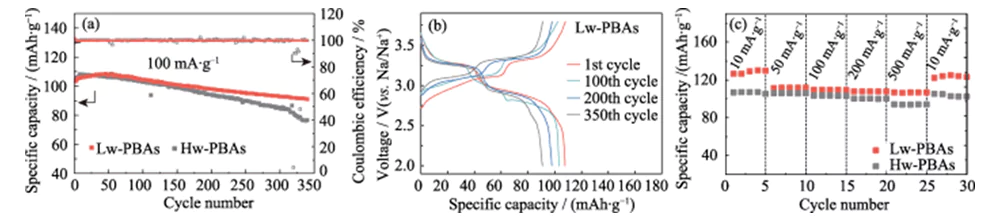
Fig. 3 Electrochemical performance of PBAs cathode in Na-ion half cell
(a) Cycling performance of Lw-PBAs and Hw-PBAs cathodes at a current density of 100 mA·g-1; (b) Charge-discharge curves of Lw-PBAs cathode at 100 mA·g-1; (c) Rate capability of Lw-PBAs and Hw-PBAs cathodes at various current densities from 10 mA·g-1 to 500 mA·g-1; The voltage window is 2.0-3.8 V (vs. Na/Na+) for all half-cell tests; Colorful figures are available on website
2.3 In-situ analysis of sodium storage mechanism
The Lw-PBAs positive electrode was matched with the HC negative electrode, and a DMC/EC solution containing 1.0 mol·L-1 NaClO4 and 5.0% FEC by mass was used as the liquid electrolyte (LE) to assemble a full battery (Lw-PBAs|LE|HC, Figure 4(a)). The dynamic structural changes of the positive and negative electrode materials of the full battery during the charge and discharge reactions were studied using in-situ analysis technology. The in-situ XRD analysis of the Lw-PBAs positive electrode showed that after the charging voltage was increased to 3.2 V, the diffraction peaks corresponding to (110) and (104) gradually merged to form a broad peak (Figure 4(b)). This phenomenon corresponds to the process of Na+ escaping from the Lw-PBAs positive electrode, causing its crystal structure to change from a trigonal structure to a cubic structure[21]. During the discharge process, no re-split of this broad peak into (110) and (104) diffraction peaks was observed, indicating that the phase change process is irreversible, resulting in the first coulombic efficiency loss. In addition, during the first charge and discharge process of the HC negative electrode, the solid electrolyte interphase (SEI) film formed on the surface leads to irreversible lithium loss (18%), which is also one of the reasons for the first coulombic efficiency loss of the whole battery (Figure 4 (c, d)).
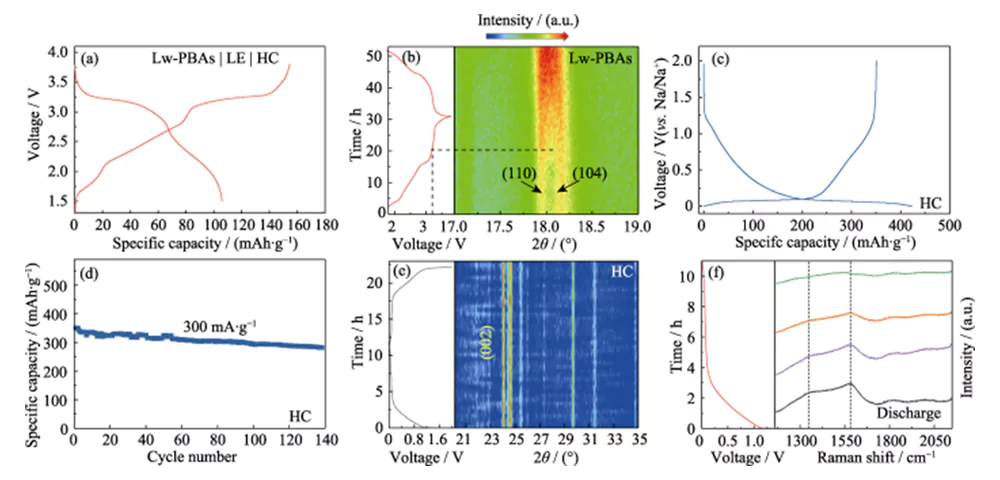
Fig. 4 In-situ analysis of Na storage mechanism for Lw-PBAs cathode and HC anode
(a) Charge-discharge curves of Lw-PBAs|LE|HC full cell; (b) In-situ XRD pattern of Lw-PBAs cathode during the operation of full cell; (c) Charge-discharge curves for the first cycle and (d) cycling stability of HC anode at a current density of 300 mA·g-1; (e) In-situ XRD pattern and (f) in-situ Raman spectra of HC anode during the operation of full cell; Colorful figures are available on website
In the in-situ XRD spectrum of the HC anode, no obvious (002) peak shift was observed during the charge and discharge process, indicating that Na+ was not inserted into the graphitized structure layers, and no diffraction peaks from sodium metal were observed (Figure 4( e)). Therefore, the sodium storage capacity of the HC anode may be due to the adsorption and filling of Na+ in the rich defect sites and pores of HC, rather than Na+ intercalation or metallic sodium precipitation [22]. In order to further study the sodium storage reaction mechanism in HC, in-situ Raman analysis was performed on the HC negative electrode during the charge and discharge process (Figure 4(f)). The HC negative electrode has obvious Raman characteristic peaks at 1350 and 1594 cm-1. The characteristic peak with a wave number of 1350 cm-1 corresponds to the aromatic carbon configuration stretching vibration (G mode), and the characteristic peak with a wave number of 1594 cm-1 corresponds to the disordered defective carbon structure (D mode). The intensity ratio of D mode and G mode (ID/IG) can be used to measure the degree of defects and disorder of carbon materials. During the discharge process, the ID/IG of the HC anode decreased with the continuous intercalation of Na+, indicating that the significant adsorption behavior of Na+ at its defect sites is the main source of the sodium storage capacity of the HC anode.
2.4 Construction and performance of quasi-solid-state full-cell
The first coulombic efficiency of the sodium-ion full-cell constructed using Lw-PBAs positive electrode and HC negative electrode is only 67.3% (Figure 4(a)). To address this problem, environmentally friendly, low-toxic, and air-stable Na2C2O4 is used as a self-sacrificial sodium compensator in the Lw-PBAs positive electrode to improve the first coulombic efficiency of the full-cell [23]. The particle size of commercial Na2C2O4 is more than hundreds of microns and has poor electrochemical activity. Therefore, it is recrystallized to obtain Na2C2O4 with a particle size of several microns (Figure 5(a)). Micron-sized Na2C2O4 can release a high specific capacity of 407 mAh·g−1 during the first charge process within the voltage window of 2.0~4.2 V, effectively compensating for the first irreversible capacity loss of the positive electrode (Figure 5(b)). The initial discharge specific capacity of the Lw-PBAs|LE|HC full cell with the addition of Na2C2O4 (mass fraction 20%) can reach 158 mAh·g-1, which is 92.7% higher than that of the full cell without the addition of Na2C2O4 (Figure 5(c)). The Lw-PBAs|LE|HC full cell with the addition of Na2C2O4 can maintain a reversible specific capacity of 110, 101, 92, 87 and 80 mAh·g-1 at current densities of 10, 50, 100, 200 and 500 mA·g-1 (Figure 5(d)). At a high current density of 500 mA·g-1, after 1400 stable cycles, the Lw-PBAs|LE|HC full cell with the addition of Na2C2O4 can maintain a specific capacity of 64 mAh·g-1, which is 25.4% higher than that of the full cell without the addition of Na2C2O4 (Figure 5(e)).
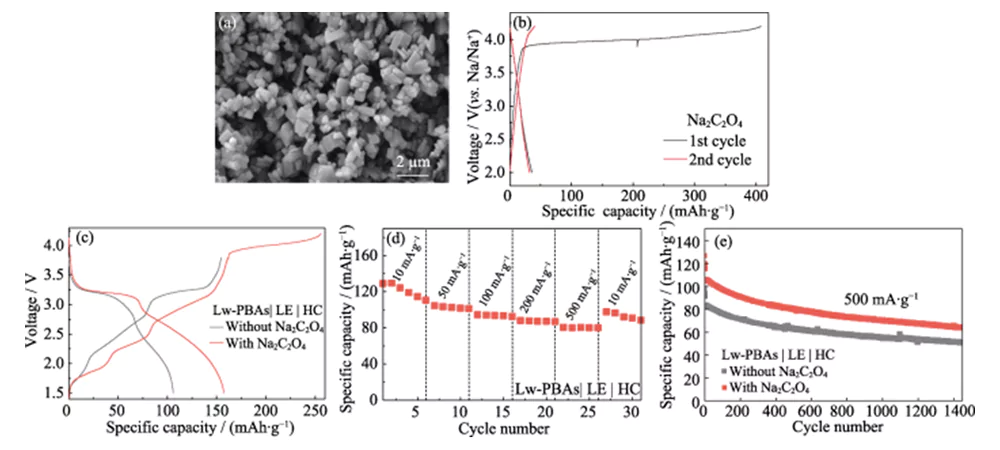
Fig. 5 Effect of Na2C2O4 on the electrochemical performance of Lw-PBAs cathode
(a) SEM image and (b) charge-discharge curves of Na2C2O4 with micrometer size at a current density of 180 mA·g-1; (c) Charge-discharge curves of Lw-PBAs|LE|HC full cells with or without adopting Na2C2O4 at a current density of 100 mA·g-1; (d) Rate performance of Lw-PBAs|LE|HC full cell with Na2C2O4 at various current densities from 10 to 500 mA·g-1; (e) Cycling stability of Lw-PBAs|LE|HC full cell with or without using Na2C2O4 at large current density of 500 mA·g-1; The voltage window is 1.5-3.8 V for all full-cell tests; Colorful figures are available on website
On this basis, PEGDA was mixed with 1.0 mol·L-1 NaClO4 and DMC/EC electrolyte with a mass fraction of 5.0% FEC, and AIBN was used as a thermal polymerization initiator to develop a high-performance quasi-solid electrolyte (GPE). Compared with LE, GPE has the advantages of being less prone to leakage and low volatility. It can remain stable at a high voltage of 4.9 V (vs. Na/Na+) and has a wide electrochemical stability window (Figure 6(a)). Compared with solid electrolytes, GPE has higher ionic conductivity and interface compatibility, and the room temperature ionic conductivity is 3.51 mS·cm-1 (Figure 6(b)). It was further matched with the low-water-content Lw-PBAs positive electrode and HC negative electrode to construct a quasi-solid-state sodium-ion full battery (Lw-PBAs|GPE|HC). At a current density of 100 mA·g-1, the first discharge specific capacity of the Lw-PBAs|GPE|HC quasi-solid-state battery reached 147.8 mAh·g-1 (Figure 6(c)). At current densities of 20, 50, 100, 200 and 500 mA·g-1, the specific capacities can be maintained at 105, 94, 82, 70 and 58 mAh·g-1 (Figure 6(d)). At a current density of 100 mA·g-1, it can be stably cycled for more than 200 times, and the Coulombic efficiency is close to 100% (Figure 6(e)).
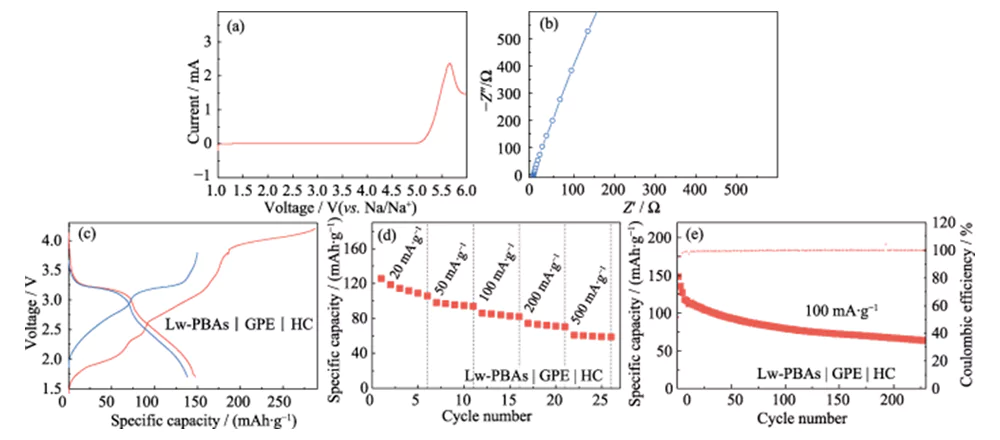
Fig. 6 Electrochemical performance of quasi-solid-state full cell based on Lw-PBAs cathode and PEGDA-based GPE
(a) LSV curve at a scan rate of 5 mV·s-1; (b) EIS spectrum; (c) Charge-discharge curves at a current density of 100 mA·g-1; (d) Rate performance at current densities of 20-500 mA·g-1; (e) Cycling performance at 100 mA·g-1; The voltage window is 1.5-3.8 V for all full-cell tests
3 Conclusion
In this study, low-water content PBAs cathode materials were prepared by a simple and efficient heat treatment method. It was found that the removal of crystal water not only increased the capacity retention rate of the PBAs cathode from 73% to 88% after 340 cycles, but also helped to strengthen the redox reaction of low-spin Fe2+/Fe3+ in PBAs, thereby improving its sodium storage capacity. The dynamic sodium storage mechanism of PBAs cathode and HC anode was revealed by in situ Raman and in situ XRD techniques. The analysis showed that the process of Na+ escaping from the PBAs cathode caused its crystal structure to irreversibly change from three-dimensional cubic, resulting in the loss of the first coulombic efficiency, and the adsorption of Na+ at its defect sites was the main source of the sodium storage capacity of the HC anode. After adding Na2C2O4 sodium compensator (mass fraction 20%) to the cathode, the first discharge capacity of the PBAs cathode increased by 92.7%. Based on the thermal polymerization of PEGDA initiated by AIBN, a high-performance quasi-solid electrolyte with room temperature ionic conductivity of 3.51 mS·cm-1 and electrochemical stability window widened to 4.9 V (vs. Na/Na+) was developed. On this basis, a low-water-content PBAs cathode with added Na2C2O4 sodium compensator, HC anode and PEGDA benchmark solid electrolyte were integrated to construct a quasi-solid-state sodium-ion battery that can be stably cycled for more than 200 times at a current density of 100 mA·g-1. Studies have shown that efficient removal of crystal water is a necessary means to improve the cycle stability of PBAs cathode and realize the creation of high-performance quasi-solid-state sodium-ion batteries.
[1] WANG W L, GANG Y, PENG J, et al. Effect of eliminating water in Prussian blue cathode for sodium-ion batteries. Adv. Funct. Mater., 2022, 32(25): 2111727.
[2] MENG X Y, LIU Y Z, WANG Z Y, et al. A quasi-solid-state rechargeable cell with high energy and superior safety enabled by stable redox chemistry of Li2S in gel electrolyte. Energy Environ. Sci., 2021, 14(4): 2278.
[3] CHE H Y, CHEN S L, XIE Y Y, et al. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci., 2017, 10(5): 1075.
[4] LI W K, ZHAO N, BI Z J, et al. Na3Zr2Si2PO12 ceramic electrolytes for Na-ion battery: preparation using spray-drying method and its property. J. Inorg. Mater., 2022, 37(2) : 189.
[5] LI D, LEI C, LAI H, et al. Recent advancements in interface between cathode and garnet solid electrolyte for all solid state Li-ion batteries. J. Inorg. Mater., 2019, 34(7): 694.
[6] KIM K J, BALAISH M, WADAGUCHI M, et al. Solid-state Li-metal batteries: challenges and horizons of oxide and sulfide solid electrolytes and their interfaces. Adv. Energy Mater., 2021, 11(1): 2002689.
[7] GAO H, GUO B, SONG J, et al. A composite gel-polymer/glassfiber electrolyte for sodium-ion batteries. Adv. Energy Mater., 2015, 5(9): 1402235.
[8] LIU Y Z, MENG X Y, SHI Y, et al. Long-life quasi-solid-state anode-free batteries enabled by Li compensation coupled interface engineering. Adv. Mater., 2023, 35(42): e2305386.
[9] DU G Y, TAO M L, LI J, et al. Low-operating temperature, highrate and durable solid-state sodium-ion battery based on polymer electrolyte and Prussian blue cathode. Adv. Energy Mater., 2020, 10(5): 1903351.
[10] PENG J, ZHANG W, LIU Q N, et al. Prussian blue analogues for sodium-ion batteries: past, present, and future. Adv. Mater., 2022, 34(15): 2108384.
[11] LU Y H, WANG L, CHENG J G, et al. Prussian blue: a new framework of electrode materials for sodium batteries. Chem. Commun., 2012, 48(52): 6544.
[12] SÅNGELAND C, MOGENSEN R, BRANDELL D, et al. Stable cycling of sodium metal all-solid-state batteries with polycarbonatebased polymer electrolytes. ACS Appl. Poly. Mater., 2019, 1(4): 825.
[13] KIM T, AHN S H, SONG Y Y, et al. Prussian blue-type sodium-ion conducting solid electrolytes for all solid-state batteries. Angew. Chem. Int. Ed., 2023, 62(42): e202309852.
[14] SONG J, WANG L, LU Y H, et al. Removal of interstitial H2O in hexacyanometallates for a superior cathode of a sodium-ion battery. J. Am. Chem. Soc., 2015, 137(7): 2658.
[15] LIU Y, FAN S, GAO Y, et al. Isostructural synthesis of iron-based Prussian blue analogs for sodium-ion batteries. Small, 2023, 19(43): e2302687.
[16] WANG W, GANG Y, HU Z, et al. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat. Commun., 2020, 11: 980.
[17] YOU Y, YU X Q, YIN Y X, et al. Sodium iron hexacyanoferrate with high Na content as a Na-rich cathode material for Na-ion batteries. Nano Res., 2014, 8(1): 117.
[18] REN W H, QIN M S, ZHU Z X, et al. Activation of sodium storage sites in Prussian blue analogues via surface etching. Nano Lett., 2017, 17(8): 4713.
[19] ZHANG H, GAO Y, PENG J, et al. Prussian blue analogues with optimized crystal plane orientation and low crystal defects toward 450 Wh·kg−1 alkali-ion batteries. Angew. Chem. Int. Ed., 2023, 62(27): e202303953.
[20] ZHANG Z H, AVDEEV M, CHEN H C, et al. Lithiated Prussian blue analogues as positive electrode active materials for stable non-aqueous lithium-ion batteries. Nat. Commun., 2022, 13: 7790.
[21] JIANG M, HOU Z, MA H, et al. Resolving deactivation of low-spin Fe sites by redistributing electron density toward highenergy sodium storage. Nano Lett., 2023, 23(22): 10423.
[22] TANG Z, ZHANG R, WANG H Y, et al. Revealing the closed pore formation of waste wood-derived hard carbon for advanced sodium-ion battery. Nat. Commun., 2023, 14: 6024.
[23] NIU Y B, GUO Y J, YIN Y X, et al. High-efficiency cathode sodium compensation for sodium-ion batteries. Adv. Mater., 2020, 32(33): e2001419.





